Reserving COVID-19 Vaccines for Global Access: Cross-Sectional Analysis
This Table and these Figures accompany the BMJ manuscript, “Reserving COVID-19 Vaccines for Global Access: Cross-Sectional Analysis,” found here by Anthony D. So (Professor of the Practice, Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, United States of America; Director, Innovation + Design Enabling Access (IDEA) Initiative, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, United States of America) and Joshua Woo (Research Assistant, Innovation + Design Enabling Access (IDEA) Initiative, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, United States of America). All data are as of November 15, 2020. The full supplemental file can be downloaded here.
We graciously acknowledge the support of this work from the Innovation + Design Enabling Access (IDEA) Initiative, Johns Hopkins Bloomberg School of Public Health, the Johns Hopkins Alliance for a Healthier World, and the Open Society Foundation.
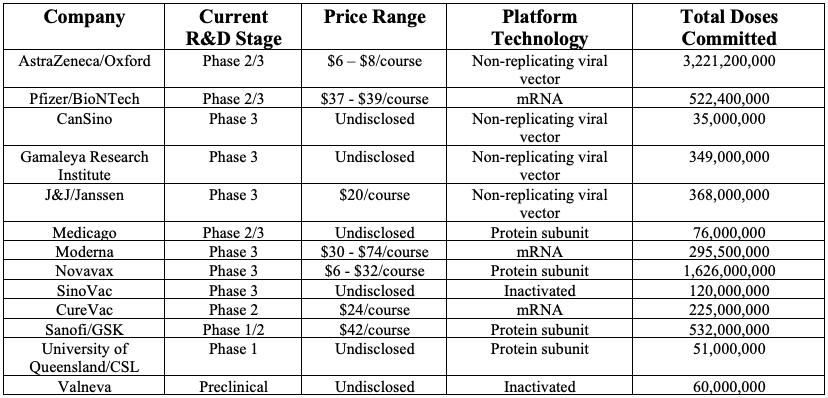
Table 1. COVID-19 Vaccines: Overview of leading companies with pre-market purchase commitments
Figure S1. COVID-19 Vaccines: Who has reserved them and where might they be going?
This Sankey diagram maps the flows of publicly known, COVID-19 vaccines pre-market purchase commitments from those who have reserved them, the vaccine manufacturers supplying these doses, the tiered pricing of these vaccine doses, and their potential recipient countries. This visualization was made using Flourish.
Notes:
Under a $300M grant from the Bill and Melinda Gates Foundation, the Serum Institute of India will scale up production of 200 M vaccine doses from AstraZeneca/Oxford University for 57 COVAX AMC-eligible LMIC countries and Novavax for 92 AMC-eligible LMIC countries at a ceiling price of $3/dose or $6/course. The precise allocation of these 200 M vaccine doses between the two firms is not yet known. For purposes of this study and the data visualization, the volume of this pre-market purchase commitment is divided equally between the two vaccine manufacturers.
Two licensing agreements with the Serum Institute of India--one with AstraZeneca/Oxford University and Novavax--each carry the potential of delivering a billion doses. It has been assumed that these doses, destined for use in low- and middle-income countries, would be made available at the ceiling price of $3 per dose, or $6 per course, set by the separate Bill and Melinda Gates Foundation grant with the Serum Institute of India for both of these manufacturers. However, this pricing information remains to be publicly confirmed.
Only publicly disclosed deals are visualized in this diagram; however, the vaccine price is not always known. These are labeled as “Undisclosed Price in the third pillar of the Sankey diagram.
The European Inclusive Vaccines Alliance, comprised of France, Italy, Germany and the Netherlands, has secured COVID-19 vaccines for countries in the European Union and beyond. In this data visualization, it is assumed that its pre-market purchase commitments will go to the European Union.
Not included in this visualization are the 200 million doses of Sanofi/GSK’s protein based COVID-19 vaccine for which the COVAX Facility has reached an agreement signaling its intention to purchase.
Figure S2. Companies bringing forward COVID-19 vaccine candidates with leading pre-market purchase commitments
This Sankey diagram maps the flows of publicly known, COVID-19 vaccines pre-market purchase commitments from companies with leading pre-market purchase commitments. This visualization was made using Flourish.
Notes:
Under a $300M grant from the Bill and Melinda Gates Foundation, the Serum Institute of India will scale up production of 200 M vaccine doses from AstraZeneca/Oxford University for 57 COVAX AMC-eligible LMIC countries and Novavax for 92 AMC-eligible LMIC countries at a ceiling price of $3/dose or $6/course. The precise allocation of these 200 M vaccine doses between the two firms is not yet known. For purposes of this study and the data visualization, the volume of this pre-market purchase commitment is divided equally between the two vaccine manufacturers.
Two licensing agreements with the Serum Institute of India--one with AstraZeneca/Oxford University and Novavax--each carry the potential of delivering a billion doses. It has been assumed that these doses, destined for use in low- and middle-income countries, would be made available at the ceiling price of $3 per dose, or $6 per course, set by the separate Bill and Melinda Gates Foundation grant with the Serum Institute of India for both of these manufacturers. However, this pricing information remains to be publicly confirmed.
Only publicly disclosed deals are visualized in this diagram; however, the vaccine price is not always known. These are labeled as “Undisclosed Price in the third pillar of the Sankey diagram.
Not included in this visualization are the 200 million doses of Sanofi/GSK’s protein based COVID-19 vaccine for which the COVAX Facility has reached an agreement signaling its intention to purchase.
Figure 1. Pre-Market Commitments for COVID-19 Vaccines, Per Capita (in courses)
Notes:
1. Vaccine courses are assumed to require two doses, except for CanSino which proposes to be one-dose per course. Johnson & Johnson/Janssen’s vaccine candidate is testing for both a one-dose and two-dose course, but for purposes of this study’s analysis, is assumed to be a two-dose course vaccine.
2. The vaccine courses for the European Union include pre-market purchase commitments not only by the European Union, but also by the European Inclusive Vaccines Alliance.
Figure 2. Projected Manufacturing Capacity by Lead COVID-19 Vaccine Companies by End of 2021
Note: Vaccine courses are assumed to require two doses, except for CanSino which proposes to be one-dose per course. Johnson & Johnson/Janssen’s vaccine candidate is testing for both a one-dose and two-dose course, but for purposes of this study’s analysis, is assumed to be a two-dose course vaccine.
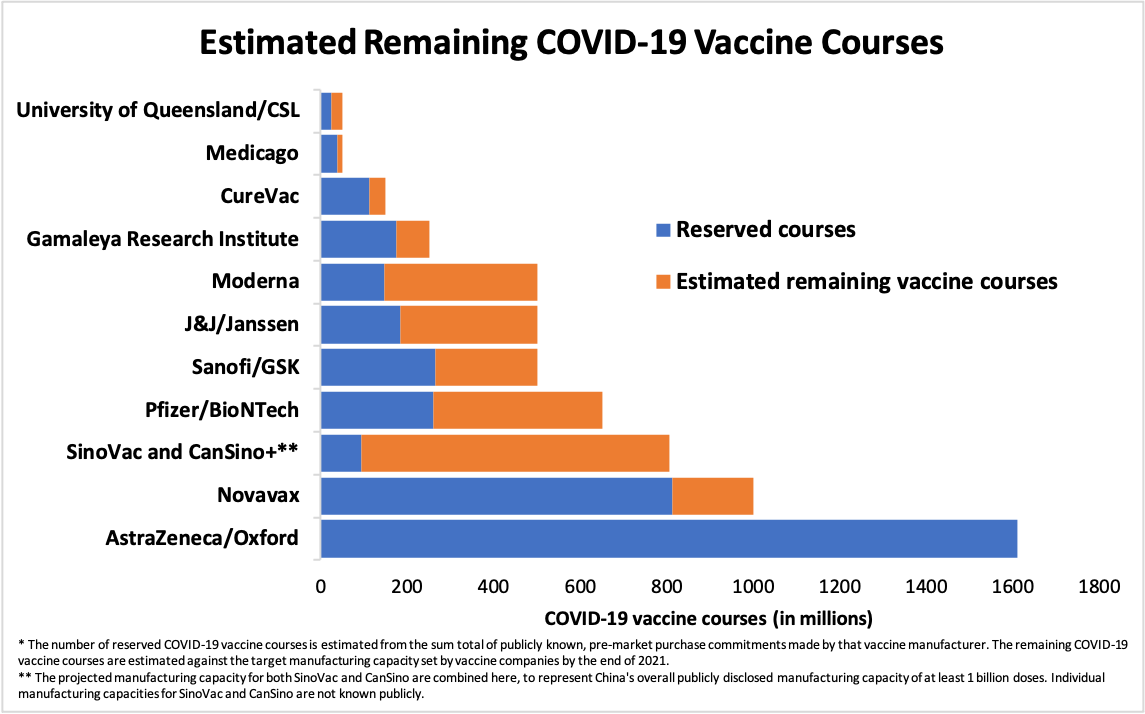
Figure 3. Estimated Remaining COVID-19 Vaccine Courses
Notes:
1. The data behind Figure 4b can be found in the Supplemental Data, Table S4.
2. The production capacity attributed to SinoVac, CanSino and other Chinese vaccine manufacturers is assumed to be part of the projected national vaccine production capacity announced by China’s National Health Commission for 2020 and 2021.
We welcome those viewing this site to share any additional information or insights they may have. We are making this information freely available, without any warranty nor any assumption of liability for its use. If there is any missing or incorrect information, please forward this to ignitetheidea@gmail.com.